The Carbon Molecule
Carbon is found in many different compounds. It is in the food you eat, the clothes you wear, the cosmetics you use and the gasoline that fuels your car. In addition, carbon is a very special element because it plays a dominant role in the chemistry of life.
The element carbon
Carbon has four electrons in its valence shell (outershell). Since this energy shell can hold eight electrons, each carbon atom can share electrons with up to four different atoms. Carbon can combine with other elements as well as with itself. This allows carbon to form many different compounds of varying size and shape.
Carbon alone forms the familiar substances graphite and diamond. Both are made only of carbon atoms. Graphite is very soft and slippery. Diamond is the hardest substance known to man. If both are made only of carbon what gives them different properties? The answer lies in the way the carbon atoms form bonds with each other.
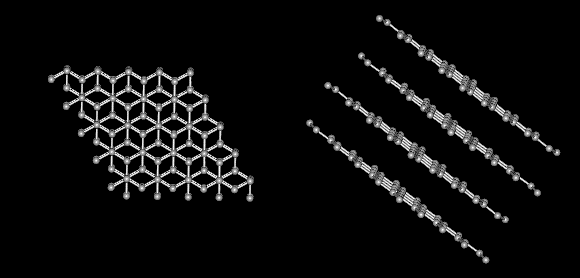
Notice that graphite is layered.
There are strong covalent bonds between carbon atoms in each layer. But, only weak forces exist between layers. This allows layers of carbon to slide overeach other in graphite.
On the other hand, in diamond each carbon atom is the same distance to each of its neighboring carbon atoms. In this rigid network atoms cannot move. This explains why diamonds are so hard and have such a high melting point.
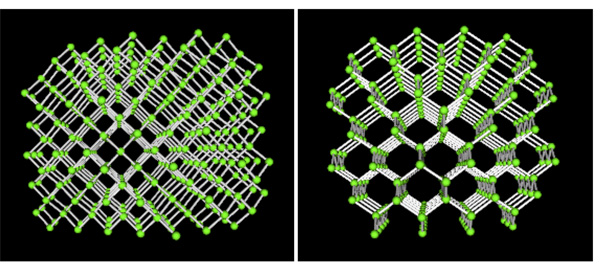
For 3D structures and images of carbon molecules using Jsmol - (graphite, diamond and fullerene) see: Interactive Molecules Page
The 3-D coordinates for graphite and diamond are available in the EDinformatics 3D Structural Database. We urge you to download these structures to your home computer and use one of the suggested 3-D viewers. See Free Molecular Modeling Software.
The Molecule of the Month Page has recently included information on diamond located here.
A third class of carbon compounds has recently been discovered. They are called fullerenes. The figure shown below on the left is one form composed of 60 carbons. Notice the geometric patterns of pentagons and hexagons that form the familiar icosohedron.
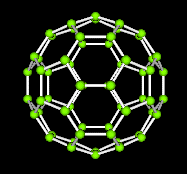
A java applet that shows the fullerene and allows rotation of the image is now available here.
Compounds made of Carbon
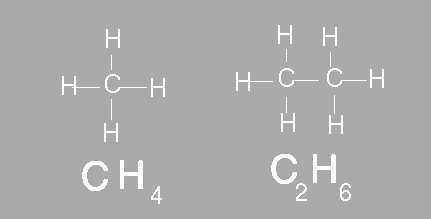
The simplest organic compounds contain molecules composed of carbon and hydrogen. The compound methane contains one carbon bonded to four hydrogens. Ethane is another example of a simple hydrocarbon. Ethane contains two carbon atoms and six hydrogen atoms. In chemistry we use a molecular formula to show how many atoms of each element are present in a molecule. A molecular formula however does not show the structure of the molecule. Scientists often use structural formulas to show the number and arrangement of atoms in a compounds. Below the molecular formula for methane and ethane are shown. Above the molecular formula are their respective structural formula.
Although structural formulas can be very helpful they do not give a complete picture of a molecule. Structural formulas do not tell us anything about the distances between bonds, the angles formed by these bonds, or the size and shape of the molecule. Scientists use three different representation to show what molecules look like.
THE WIRE FRAME MODEL
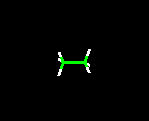
This model clearly shows the type of atoms in the molecule, the distances between bonds, and angles associated with the atoms. Because the lines drawn are very thin, molecules can very easily be manipulated when viewed on a computer screen.
THE BALL-AND-STICK MODEL
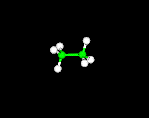
Atoms are represented by balls and bonds are represented as sticks.
THE SPACE FILLED MODEL
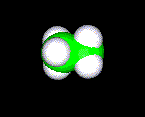
This model shows the space that the molecule will take up. Because of all the points required to draw this molecule on a computer screen you should expect these molecules to be very difficult to manipulate.
Carbon Atoms and Molecules
- CARBON MODULE HOME
- THE CARBON ATOM
- CABON MOLECULE
- CARBON THE ELEMENT
- SIMPLE CARBON COMPOUNDS
- CARBON CHEMISTRY JMOL
- CARBON CHEMISTRY JSMOL
- GRAPHENE MOLECULE
- HOW BIG IS A BUCKEYBALL
- FULLERENE MOLECULE
- GRAPHITE AND DIAMOND
- GRAPHITE AND CARBYNE
- CARBON MONOXIDE
- GEOMETRY OF METHANE
- BENZENE MOLECULE
- CARBON FUELS
