What
is the Glucosamine/chondroitin Arthritis Intervention
Trial (GAIT)?
GAIT is the first large-scale, multicenter clinical trial
in the United States to test the effects of the dietary
supplements glucosamine hydrochloride (glucosamine) and
sodium chondroitin sulfate (chondroitin sulfate) for the
treatment of knee osteoarthritis. The study tested whether
glucosamine and chondroitin sulfate used separately or
in combination reduced pain in participants with knee
osteoarthritis.
The University of Utah, School of Medicine coordinated this study, which was conducted at 16 rheumatology research centers across the United States. The National Center for Complementary and Alternative Medicine (NCCAM) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), two components of the National Institutes of Health (NIH), funded GAIT.
What
was the purpose of the study?
Previous studies in the medical literature had conflicting
results on the effectiveness of glucosamine and chondroitin
sulfate as treatments for osteoarthritis. GAIT was designed
to test the short-term (6 months) effectiveness of glucosamine
and chondroitin sulfate in reducing pain in a large number
of participants with knee osteoarthritis.
What
was the basic design of the study?
In GAIT, participants were randomly assigned to one of
five treatment groups: (1) glucosamine alone, (2) chondroitin
sulfate alone, (3) glucosamine and chondroitin sulfate
in combination, (4) celecoxib, or (5) a placebo (an inactive
substance that looks like the study substance). Glucosamine
and chondroitin sulfate and their combination were compared
with a placebo to evaluate whether these substances significantly
improve joint pain. Celecoxib, which is a prescription
drug effective in managing osteoarthritis pain, was also
compared with placebo to validate the study design.
To reduce the chance of biased results, the study was double-blinded--neither the researchers nor the participants knew which of the five treatment groups the participants were in. Participants received treatment for 24 weeks. Participants were evaluated at the start of the study and at weeks 4, 8, 16, and 24 and closely monitored for improvement of their symptoms as well as for any possible adverse reactions to the study agents. X-rays documented each participant's diagnosis of osteoarthritis. Participants were also stratified into two pain subgroups-- 1,229 participants (78 percent) with mild pain and 354 participants (22 percent) with moderate-to-severe pain.
A positive response to treatment was defined as a 20 percent or greater reduction in pain at week 24 compared to the start of the study. All participants had the option to use up to 4000 mg of acetaminophen, as needed, to control pain from osteoarthritis throughout the study, except for the 24 hours prior to having their knee assessed. Acetaminophen use was low: on average, participants used fewer than two 500 mg tablets per day.
What
did GAIT cost?
The primary GAIT study cost just over $12.5 million.
Study Background
What
is osteoarthritis?
More than 20 million adults in the United States live
with osteoarthritis--the most common type of arthritis.
Osteoarthritis, also called degenerative joint disease,
is caused by the breakdown of cartilage, which is the
connective tissue that cushions the ends of bones within
the joint. Osteoarthritis is characterized by pain, joint
damage, and limited motion. The disease generally occurs
late in life, and most commonly affects the hands and
large weight-bearing joints, such as the knees. Age, female
gender, and obesity are risk factors for this condition.
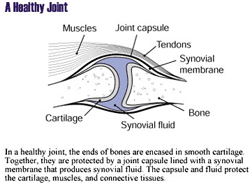 |
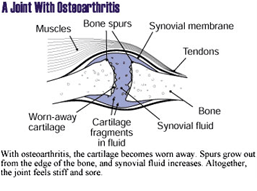 |
|
| Image provided by the National
Institute of Arthritis and Musculoskeletal and Skin
Diseases (NIAMS) |
||
What
are glucosamine and chondroitin sulfate?
Glucosamine and chondroitin sulfate are natural substances
found in and around the cells of cartilage. Glucosamine
is an amino sugar that the body produces and distributes
in cartilage and other connective tissue, and chondroitin
sulfate is a complex carbohydrate that helps cartilage
retain water. In the United States, glucosamine and chondroitin
sulfate are sold as dietary supplements, which are regulated
as foods rather than drugs.
What
is celecoxib?
Celecoxib (brand name Celebrex) is a type of nonsteroidal
anti-inflammatory drug (NSAID), called a COX-2 inhibitor.
Like traditional NSAIDs, celecoxib blocks the COX-2 enzyme
in the body that stimulates inflammation. Unlike traditional
NSAIDs, however, celecoxib does not block the action of
COX-1 enzyme, which is known to protect the stomach lining.
As a result, celecoxib reduces joint pain and inflammation
with reduced risk of gastrointestinal ulceration and bleeding.
Recent reports have linked possible cardiovascular side
effects to COX-2 inhibitors. Although GAIT was not designed
to study the safety of celecoxib, participants were monitored
for adverse events and no increase in such side effects
was observed.
What
doses were used for the various treatments?
The doses used in GAIT were based on the doses seen in
the prevailing scientific literature.
- Glucosamine alone: 1500 mg daily given as 500 mg three times a day
- Chondroitin sulfate alone: 1200 mg daily given as 400 mg three times a day
- Glucosamine plus chondroitin sulfate combined: same doses-1500 mg and 1200 mg daily
- Celecoxib: 200 mg daily
- Acetaminophen: participants were allowed to take up to 4000 mg (500 mg tablets) per day to control pain, except for the 24 hours before pain was assessed.
Who
provided the source materials for making the glucosamine
and chondroitin sulfate products used in GAIT?
- Glucosamine was donated in part by Ferro Pfanstiehl Laboratories, Inc., Waukegan, IL, through Wilke Resources.
- Chondroitin sulfate was donated by Bioiberica, S.A., Barcelona, Spain.
Where
did the other study products come from?
- Acetaminophen was donated by McNeil Consumer and Specialty Pharmaceuticals, Fort Washington, PA.
- Celecoxib was purchased from Pfizer.
Where
was the study conducted?
- University of Alabama at Birmingham, Birmingham, AL; Larry W. Moreland, M.D.
- University of Arizona, Tucson, AZ; David Yocum, M.D.
- Cedars-Sinai Medical Center, Los Angeles, CA; Michael Weisman, M.D.
- University of California Los Angeles, Los Angeles, CA; Daniel Furst, M.D.
- University of California San Francisco, San Francisco, CA; Nancy Lane, M.D.
- Northwestern University, Chicago, IL; Thomas J. Schnitzer, M.D.
- Indiana University, Indianapolis, IN; John Bradley, M.D.
- The Arthritis Research and Clinical Centers, Wichita, KS; Frederick Wolfe, M.D.
- University of Nebraska Medical Center, Omaha, NE; James O'Dell, M.D.
- Hospital for Joint Diseases, New York, NY; Clifton Bingham, III, M.D.
- Case Western Reserve University, Cleveland, OH; Michele Hooper, M.D.
- University of Pennsylvania, Philadelphia, PA; H. Ralph Schumacher, Jr., M.D.
- University of Pittsburgh, Pittsburgh, PA; Chester Oddis, M.D.
- Presbyterian Hospital of Dallas, Dallas, TX; John J. Cush, M.D.
- University of Utah, Salt Lake City, UT; Christopher G. Jackson, M.D.
- Virginia Mason Medical Center, Seattle, WA; Jerry Molitor, M.D.
Key Results
What
were the key results of the study?
Researchers found that:
- Participants taking the positive control, celecoxib, experienced statistically significant pain relief versus placebo--about 70 percent of those taking celecoxib had a 20 percent or greater reduction in pain versus about 60 percent for placebo.
- Overall, there were no significant differences between the other treatments tested and placebo.
- For a subset of participants with moderate-to-severe pain, glucosamine combined with chondroitin sulfate provided statistically significant pain relief compared with placebo--about 79 percent had a 20 percent or greater reduction in pain versus about 54 percent for placebo. According to the researchers, because of the small size of this subgroup these findings should be considered preliminary and need to be confirmed in further studies.
- For participants in the mild pain subset, glucosamine and chondroitin sulfate together or alone did not provide statistically significant pain relief.
How
many people participated in the study and who were they?
A total of 1,583 people participated in the study. People
age 40 or older with knee pain and documented x-ray evidence
of osteoarthritis were eligible to participate. Participants
could not have used glucosamine for 3 months and chondroitin
sulfate for 6 months prior to entering the study. Participants
were about 59 years of age, on average, and nearly two-thirds
of participants were women. Of the 1,583 study participants,
78 percent (1,229) were in the mild pain subgroup and
22 percent (354) were in the moderate-to-severe pain subgroup.
Were
there any side effects from the treatments?
There were 77 reports of serious adverse effects during
the study. Of those 77, only 3 were attributed to study
treatments. Most side effects were mild, such as upset
stomach, and were spread evenly across the different treatment
groups. In addition, although GAIT was not designed to
evaluate these risks, no change in glucose tolerance was
seen for glucosamine nor was an increased incidence of
cardiovascular events seen with celecoxib.
Consumer Information and Next Steps
Should
people with osteoarthritis use glucosamine and chondroitin
sulfate?
People with osteoarthritis should work with their health
care provider to develop a comprehensive plan for managing
their arthritis pain: eat right, exercise, lose excess
weight, and use proven pain medications. If people have
moderate-to-severe pain, they should talk with their health
care provider about whether glucosamine plus chondroitin
sulfate is an appropriate treatment option.
Can
U.S. consumers get the glucosamine and chondroitin sulfate
products used in GAIT?
Identical products may not be commercially available.
GAIT was conducted under an Investigational New Drug application
filed with the U.S. Food and Drug Administration (FDA).
All of the products used in the study were developed for
the study and subject to the FDA's pharmaceutical regulations.
The products were evaluated and manufactured by the VA
Cooperative Studies Program Clinical Research Pharmacy,
an FDA-licensed clinical research pharmacy center. The
glucosamine and chondroitin sulfate used were tested for
purity, potency, quality, and consistency among batches.
Products were retested for stability throughout the study.
Will
the GAIT team continue to do research on glucosamine and
chondroitin sulfate?
GAIT includes an ancillary study, which is still ongoing,
that will assess whether glucosamine and chondroitin sulfate
can reduce or halt the progression of knee osteoarthritis
following additional treatment. About one-half of the
participants enrolled in GAIT will be treated for an additional
18 months. As in the primary study, participants will
not know to which treatment group they belong. Researchers
will compare x-rays taken at the beginning of the study
and after 1 and 2 years of treatment to identify changes
in the knee joints as a result of treatment. Results are
expected in late 2007 or early 2008.
For More Information
NCCAM
Clearinghouse
The NCCAM Clearinghouse provides information on complementary
and alternative medicine (CAM) and NCCAM, including publications
and searches of Federal databases of scientific and medical
literature. The Clearinghouse does not provide medical
advice, treatment recommendations, or referrals to practitioners.
Toll-free
in the U.S.: 1-888-644-6226
TTY (for deaf and hard-of-hearing callers): 1-866-464-3615
Web site: nccam.nih.gov
E-mail: info@nccam.nih.gov
National
Institute of Arthritis and Musculoskeletal and Skin Diseases
(NIAMS), NIH
NIAMS supports research into the causes, treatment, and
prevention of arthritis and musculoskeletal and skin diseases;
the training of scientists; and the sharing of research-based
information.
Web
site: http://www.niams.nih.gov/
Toll-free in the U.S.: 1-877-22-NIAMS
Office
of Dietary Supplements (ODS), NIH
ODS seeks to strengthen knowledge and understanding of
dietary supplements by evaluating scientific information,
supporting research, sharing research results, and educating
the public. Its resources include publications and the
International Bibliographic Information on Dietary Supplements
database.
Web
site: http://ods.od.nih.gov/
E-mail: ods@nih.gov
U.S.
Food and Drug Administration (FDA)
The FDA oversees the safety of many products, such as
foods (including dietary supplements), medicines, medical
devices, and cosmetics.
Web
site: http://www.fda.gov/
Toll-free in the U.S.: 1-888-463-6332
This publication is not copyrighted and is in the public domain. Duplication is encouraged.
|
NCCAM has provided this material for your information. It is not intended to substitute for the medical expertise and advice of your primary health care provider. We encourage you to discuss any decisions about treatment or care with your health care provider. The mention of any product, service, or therapy is not an endorsement by NCCAM. |
