|
The
past few years have brought some encouraging studies to the
forefront in Alzheimer's and Dementia with several drugs in
the pipeline that show promise
Currently
available drugs for Alzheimers
See
Also -- Latest
News Updates on Drugs for Alzheimer's Disease
Four medications
are currently approved by regulatory agencies such as the US
Food and Drug Administration (FDA) and the European Medicines
Agency (EMEA) to treat the cognitive manifestations of AD: three
are acetylcholinesterase inhibitors and the other is memantine,
an NMDA receptor antagonist. No drug has an indication for delaying
or halting the progression of the disease.
|
Drug
Name
|
Molecular
Structure
|
Mechanism
of Action
|
Use
|
| Aricept®
(generic name: donepezil) Razadyne®, formerly known as Reminyl
(generic name: galantamine) |
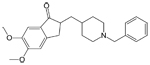 |
acetylcholinesterase
inhibitor --Prevents the breakdown of acetylcholine in the
brain |
For
people with mild ,moderate or severe AD |
| Exelon®
(generic name: rivastigmine) |
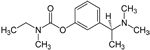 |
acetylcholinesterase
inhibitor --Prevents the breakdown of acetylcholine and
butyrylcholine (a brain chemical similar to acetylcholine)
in the brain |
For
people with mild or moderate AD |
| Razadyne®,
formerly known as Reminyl (generic name: galantamine) |
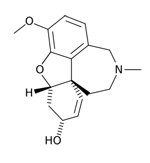 |
acetylcholinesterase
inhibitors--Prevents the breakdown of acetylcholine and
stimulates nicotinic receptors to release more acetylcholine
in the brain |
For
people with mild or moderate AD |
| Memantine
--Memantine is marketed under the brands Axura and Akatinol
by Merz, Namenda by Forest, Ebixa and Abixa by Lundbeck
and Memox by Unipharm |
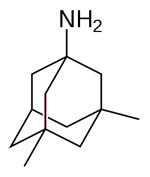 |
acting
on the glutamatergic system by blocking NMDA glutamate receptors
--Blocks the toxic effects associated with excess glutamate
and regulates glutamate activation |
Used
to treat moderate to severe AD |
Note: Cognex
(generic name: tacrine) also an acetylcholinesterase inhibitors
is not commonly used because of a number of side effects.
Cholinesterase
inhibitors are the most widely used drugs for Alzheimer's disease.
Cholinesterase inhibitors stop the breakdown of acetylcholine,
a chemical in the brain used for memory and other mental functions.
These types of medications help increase the levels of acetylcholine.
In Alzheimer’s disease, there is a deficiency in acetlycholine
in some areas of the brain, which accounts for some of the symptoms
of the disease.
It is important
to remember that these medications only slow the progression
of dementia and Alzheimer's disease – they do not stop or reverse
their course. These medications typically help for only months
to a few years and may not work as well once the disease progresses.
In general, individuals who use cholinesterase inhibitors experience
few side effects. The most commonly-experienced side effects
are gastrointestinal problems, such as nausea, diarrhea, vomiting,
and loss of appetite.
Source:
http://www.nia.nih.gov/Alzheimers/Publications/medicationsfs.htm
Research
Strategies
Intervention
strategies Researchers in Alzheimer's disease have identified
several strategies as possible interventions against amyloid:
Beta-Secretase
inhibitors. These work to block the first cleavage of APP
outside of the cell. *
Gamma-Secretase
inhibitors (e. g. Semagacestat). These work to block
the second cleavage of APP in the cell membrane and would then
stop the subsequent formation of Aß and its toxic fragments.
Selective
Aß42 lowering agents (e. g. Tarenflurbil). These modulate
gamma-secretase to reduce Aß42 production in favor of other
(shorter) Aß versions. *
Immunotherapies.
These stimulate the host immune system to recognize and attack
Aß or provide antibodies that either prevent plaque deposition
or enhance clearance of plaques.
Anti-aggregation
agents.These prevent Aß
fragments from aggregating or clear aggregates once they are
formed. There is some indication that supplementation of the
hormone melatonin may be effective against amyloid.
______________
Drugs
in the Pipeline for Alzheimers
A
variety of clinical research trials are underway with agents
that try either to decrease the amount of Aß1-42 produced or
increase the amount of Aß1-42 removed. It is hoped that such
therapies may slow down the rate of progression of Alzheimer's
disease.
| |
Molecular
Structure |
Mechanism
of Action |
Clinical
Trials |
| Bapineuzumab--Elan
and Wyeth |
This
is a Monoclonal Antibody |
Bapineuzumab
is an antibody to the beta-amyloid plaques
Note:
phase II trial, which found that bapineuzumab failed
to improve cognitive function in a test of 234 Alzheimer’s
patients after 18 months of treatment. |
PIII--Bapineuzumab
in Patients With Mild to Moderate Alzheimer's Disease
(ApoE4 Non-Carrier) -- Estimated Completion Dec.
2010
2014
: Bapineuzumab
did not improve clinical outcomes in patients
with Alzheimer's disease, despite treatment differences
in biomarkers observed in APOE e4 carriers |
|
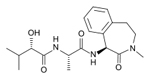
Semagacestat
LY451039
-- Elli Lilly |
|
Gamma
secretase inhibitor-- These work to block the second
cleavage of APP in the cell membrane and would then
stop the subsequent formation of amyloid --Semagacestat
blocks the enzyme gamma-secretase which is responsible
for APP proteolysis |
Phase
III --Effect of Gamma-Secretase Inhibition on
the Progression of Alzheimer's Disease: LY450139 Versus
Placebo
in August 2010, a disappointing interim
analysis, in which semagacestat performed worse than
the placebo, led to the trials being stopped |
|
Solanezumab
(Eli
Lilly) |
This
is a Monoclonal Antibody |
Solanezumab
is a monoclonal antibody that binds specifically to
soluble amyloid beta and thereby alters the aggregating
characteristics of this peptide. |
Phase
III Data set to be released in 2012
Solanezumab,
a humanized monoclonal antibody that binds amyloid,
failed
to improve cognition or functional ability. |
| Gantenerumab |
This
is a Monoclonal Antibody |
Gantenerumab
is a monoclonal antibody that is currently being evaluated
in a prodromal Alzheimer's Disease population |
Gantenerumab: a novel human anti-Aß
antibody demonstrates sustained cerebral amyloid-ß
binding and elicits cell-mediated removal of human amyloid-ß.
See
2012 study.. see
current study |
| Dimebon
-latrepirdine --from Medivation |
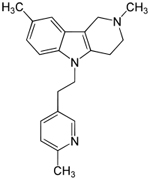 |
This
drug is an antihistamine used for 25 years in Russia--- |
Phase
III- A Phase 3 Efficacy Study Of Dimebon In Patients
With Moderate To Severe Alzheimer's Disease --
Medivation's
Dimebon fails Phase III for Alzheimer's |
| Flurizan
(*) Myriad
generic
name tarenflurbil --“enantiomer,” or mirror-image
molecule, of the non-steroidal anti-inflammatory drug
flurbiprofen |
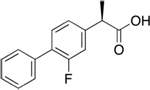 |
Lowers toxic Aß42 production by selectively modulating,
but not inhibiting, gamma-secretase activity to shift
cleavage of amyloid precursor protein (APP) away from
Aß42 production toward shorter, less toxic peptide fragments.
This drug failed a PIII trial. |
Fails
PIII -- See
Another Alzheimer’s Drug Fails in Large-Scale Trials |
| rember™--
Tau Aggregation Inhibitor (First and Second Generation)
-- TauRx |
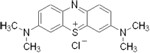 |
blocks the formation of Tau oligomers -- ability to
dissolve the tau fibers -- |
Phase
II completed.TRx0014
in Patients With Mild or Moderate Alzheimer's Disease
Phase
II - Ongoing -Open
Label Study of TRx0014 in Alzheimer's Disease
Updated
Trial |
PBT2
8-Hydroxyquinoline derivative |
 Note:
Image of 8-Hydroxyquinoline
Note:
Image of 8-Hydroxyquinoline |
targets
metal-induced aggregation of Aß, |
Completed
a Phase
IIa study in early Alzheimer's Disease patients
and has demonstrated safety and tolerability and showed
improvement in executive function
Plans
for PBT2 to Advance to Phase IIb |
Note:
Why did Flurizan fail? In the past several years, evidence has
mounted that amyloid-beta-42, long considered the culprit in
the disease, affects memory-related functions only when it has
formed multi-protein conglomerations called “oligomers.” In
the light of this concept, it is possible that Flurizan affects
amyloid-beta-42 production in the brain and reduces the formation
of insoluble amyloid deposits but has little or no effect
on amyloid oligomer levels.
Initially,
it was thought that the insoluble amyloid plaques were the pathologic
culprits in AD. However, emerging evidence implicates soluble
Aß aggregates as the mediators of neurotoxicity. The Aß rapidly
aggregates by two separate pathways. The first leads to soluble
oligomers, referred to as Aß-derived diffusible ligands (ADDLs),
referred to as ADDLs. In a separate pathway, monomers can also
form protofibrils that eventually generate fibrillar aggregates
that coalesce into the characteristic insoluble amyloid. Several
lines of in vivo evidence suggest that ADDLs and protofibrils
, rather than monomeric Aß or insoluble amyloid plaques, mediate
neurotoxicity.
For
more information and References see: Alzheimer's
Update
|

