Water has important
effects on all biological systems. What makes water so unique are two very important
properties.
Water is a polar molecule
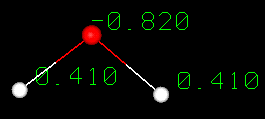 A water molecule is formed when two atoms
of hydrogen bond covalently with an atom of oxygen. In a covalent bond electrons
are shared between atoms. In water the sharing is not equal. The oxygen atom attracts
the electrons more strongly than the hydrogen.This gives water an asymmetrical
distribution of charge. Molecules that have ends with partial negative and positive
charges are known as polar molecules. It is this polar property that allows water
to separate polar solute molecules and explains why water can dissolve so many
substances.
A water molecule is formed when two atoms
of hydrogen bond covalently with an atom of oxygen. In a covalent bond electrons
are shared between atoms. In water the sharing is not equal. The oxygen atom attracts
the electrons more strongly than the hydrogen.This gives water an asymmetrical
distribution of charge. Molecules that have ends with partial negative and positive
charges are known as polar molecules. It is this polar property that allows water
to separate polar solute molecules and explains why water can dissolve so many
substances.
Water
is highly cohesive .
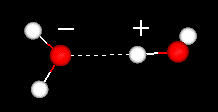 The positive regions in one water will attract the negatively charged regions
in other waters. The dashes show the hydrogen bond. In a hydrogen bond a hydrogen
atom is shared by two other atoms. The donor is the atom to which the hydrogen
is more tightly linked. The acceptor (having a partial negative charge) is the
atom which attracts the hydrogen atom. Click here or on
the image to your left to view a movie of two water molecules.
The positive regions in one water will attract the negatively charged regions
in other waters. The dashes show the hydrogen bond. In a hydrogen bond a hydrogen
atom is shared by two other atoms. The donor is the atom to which the hydrogen
is more tightly linked. The acceptor (having a partial negative charge) is the
atom which attracts the hydrogen atom. Click here or on
the image to your left to view a movie of two water molecules.
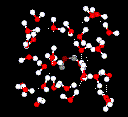 Hydrogen bonds are much weaker than covalent bonds. However, when a large number
of hydrogen bonds act in unison they will make a strong contributory effect. This
is the case in water.
Hydrogen bonds are much weaker than covalent bonds. However, when a large number
of hydrogen bonds act in unison they will make a strong contributory effect. This
is the case in water.
See: hydrogen bonds in water and ice -- Water and Ice hydrogen bonds using Jsmol
Liquid water has a partially ordered structure
in which hydrogen bonds are constantly being formed and breaking up.
See
a flash movie of water molecules in action.
On the other hand ice has a rigid
lattice structure.
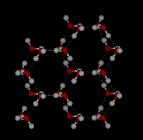
In
liquid water each molecule is hydrogen bonded to approximately 3.4 other water
molecules. In ice each each molecule is hydrogen bonded to 4 other molecules.
Compare the two structures below. Notice the empty spaces within
the ice structure.
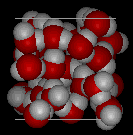
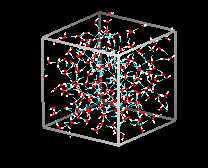
In ice Ih,
each water forms four hydrogen bonds with O---O distances of 2.76 Angstroms to
the nearest oxygen neighbor. The O-O-O angles are 109 degrees, typical of a tetrahedrally
coordinated lattice structure. The density of ice Ih is 0.931
gm/cubic cm. This compares with a density of 1.00 gm/cubic cm. for water.
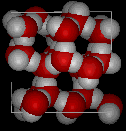
There
are eleven different forms of crystalline ice that are know. The hexaganol form
known as ice Ih is the only one that is found naturally. The lattice structure
of ice 1h is shown here.
 Image
Image

